Navigation auf uzh.ch
Navigation auf uzh.ch
Our first aim is to identify imaging markers in CT, MRI and angiography that reliably assess tissue microcirculation, collateral status as well as CVR to predict therapeutic success and outcome in stroke patients. Furthermore, we plan to characterize the interaction between these imaging markers, measures of cortical plasticity using transcranial magnetic stimulation (TMS) and functional recovery/outcome.
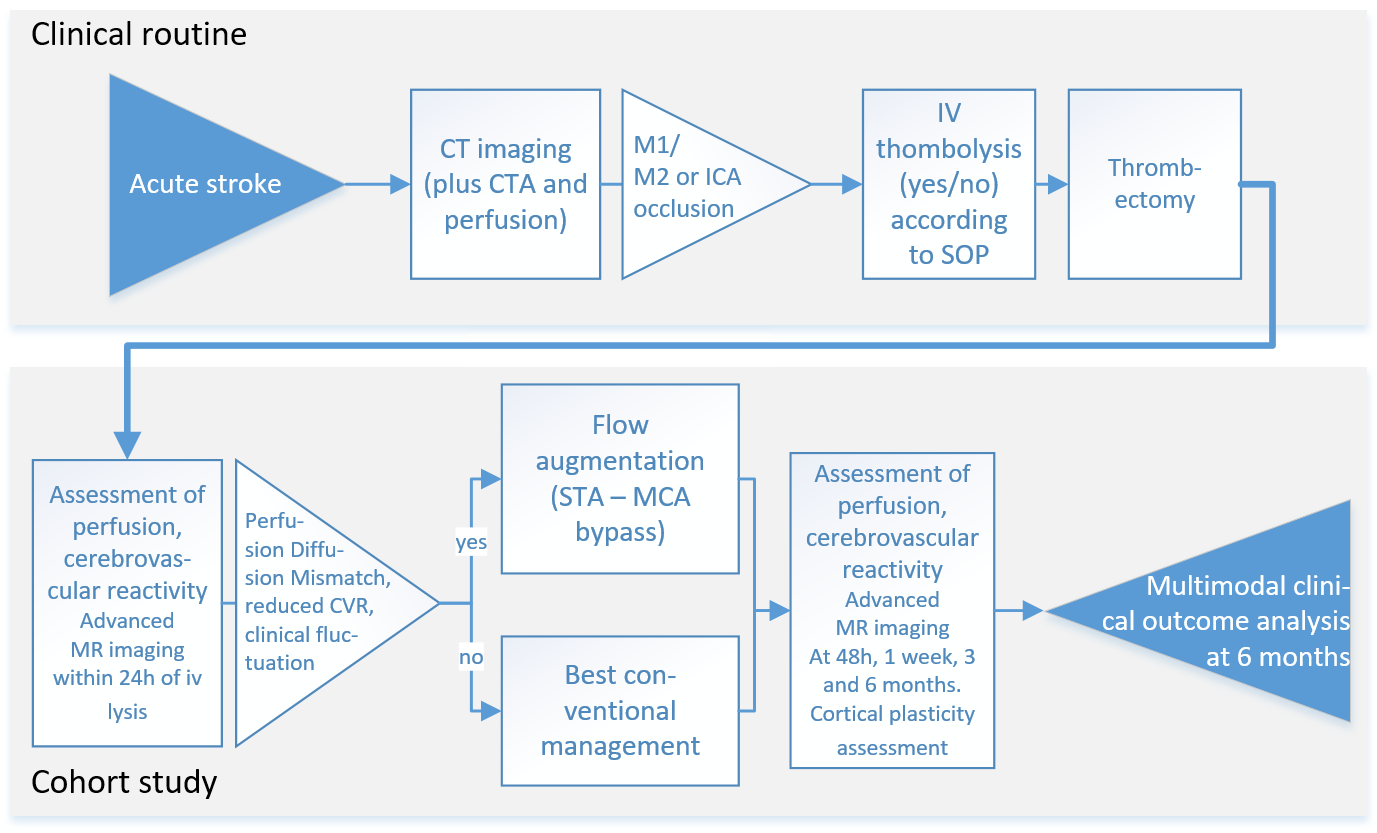
Patients with acute stroke due to large vessel occlusion will undergo standard diagnostic procedures including clinical evaluation and CT imaging of the brain. CT will include CT angiography and CT perfusion imaging. All patients eligible for thrombectomy will receive treatment according to current standards of practice and treatment success will be evaluated on the post-interventional angiography (modified thrombolysis in ischemic infarction, mTICI score). Patients who consent to the study will receive MRI of perfusion, tissue status and CVR at 24h hours, 1 week, 3 and 6 months after stroke. In addition to routine outcome assessment including the neurological function score National Institute of Health Stroke Scale (NIHSS), the functional disability score modified Rankin Scale (mRS), and a motor deficit score (Fugl Meyer Motor Assessment) will be collected. We will assess reorganization in infarct and periinfarct brain regions using transcranial magnetic stimulation (TMS) (Cantarero et al., 2013; Xu et al., 2017). Imaging the established infarct after 6 months including assessment of reperfusion at this chronic stage will complement outcome characterization.
Patients from the cohort in aim 1 who are clinically unstable (worsening or fluctuating symptoms within the initial 48h) and demonstrate significant perfusion diffusion mismatch patterns and reduced CVR will be evaluated for external-internal carotid bypass surgery (EC-IC flow augmentation bypass). The decision to perform the EC-IC bypass will not be influenced by participation in the study. The comparison of patients with and without this procedure – both undergo the same comprehensive protocol outlined in aim 1 – will provide a unique opportunity to study whether flow augmentation through bypass surgery in the acute phase can improve microcirculation and potentially recovery.
The decision for treatment modality will be based on interdisciplinary evaluation within the established clinical routines of the Clinical Neuroscience Center (Neurovascular Board) including factors such as vascular anatomy, age and comorbidities. Bypass surgery will be performed within 48 hours of stroke in those patients, in which reperfusion failure was detected by angiographic assessment and in whom a) diffusion/perfusion MRI including CVR assessment ascertains the presence of tissue at risk (DWI/PWI mismatch) as well as a reduced CVR on the ischemic hemisphere. The same clinical, imaging and TMS outcome parameters as in aim 1 will be used for follow-up until 6 months after stroke. We hypothesize that additional perfusion augmentation by bypass surgery will favorably influence tissue perfusion, plasticity measures and clinical outcomes.
Clinical observational and cohort study.