Navigation auf uzh.ch
Navigation auf uzh.ch
The consequences of ischemic stroke on quality of life can be minimized by interventions in the acute phase that aim at reestablishing circulation and tissue perfusion. As several recent clinical trials have clearly shown, recanalization of large intracranial arteries by thrombectomy improves outcome in patients with large vessel occlusion (LVO). However, not everybody benefits from recanalization and the individual benefit is difficult to predict. This variability may be caused by the fact that opening of a large vessel does not automatically improve microcirculation on the tissue level. Adequate microcirculation, however, is crucial for neurons to function and survive. Besides vessel patency, microcirculation is influenced by collateral blood supply to the territory of the occluded/recanalized large artery, by the degree of reperfusion injury, by cerebrovascular reactivity (CVR) and by blood brain barrier damage or even hemorrhage affecting ischemic tissue (Figure 1). Still largely unexplored is the interaction between microcirculation and the ability of the surviving tissue to adapt and compensate for the loss of neurons – a process referred to as neuroplasticity. Neuroplasticity occurs surrounding the infarct (periinfarct cortex) and throughout the brain. In periinfarct cortex, plasticity is enhanced – possibly a self-healing mechanism of the brain – which may also depend on effective microcirculation.
In this project, our objective is to investigate the relationship between microcirculation, collateral perfusion, CVR and neuroplasticity, the evolution of these factors over time after stroke and their predictive value for recovery. Specifically, we propose (1) to develop imaging and transcranial magnetic stimulation markers to study the interplay between microcirculation, plasticity and functional recovery, (2) to test the effect of augmenting large artery flow on microcirculation using cortical bypass surgery or endovascular revascularization in an observational clinical cohort study and (3) to investigate the mechanisms underlying microvascular reperfusion, collateral network and plasticity using a dedicated in vivo animal model of stroke and thrombolysis.
This interdisciplinary project is enabled by a close collaboration within the clinical neuroscience center Zurich between the departments of neurology, neuroradiology, neurosurgery and neuropathology. Within our collaborative network, imaging and electrophysiological modalities to study microcirculation, CVR and plasticity after stroke have already been established, and will be complemented by state-of-the-art in vivo models. The applicants’ research network has strong ties within the Zurich Neuroscience community and with international collaborators who can provide the methodological support and developments foreseen with this project.
We are convinced that this multidisciplinary, translational research approach will advance our understanding of success and failures in stroke treatment. With this and subsequent prolongations of this CRPP, our goal is to take this knowledge into a prospective clinical trial to develop imaging and treatment modalities that will directly benefit stroke patients.
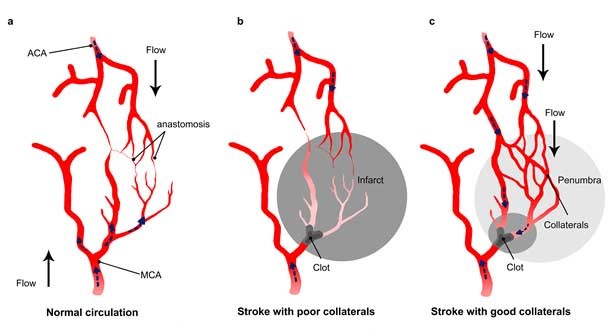
Figure 1: Impact of collateral flow on clot lysis and reperfusion.
A) Schematic draw showing anastomoses between the middle cerebral artery (MCA) and the anterior cerebral artery (ACA). B) In stroke with a poor collateral network, the collaterals fail to fill and insufficiently compensate flow reduction, while c) a collateral enhancement occurs in patients with good collaterals. The flow in the collaterals changes direction and allows the thrombolytic drug to reach the clot from different side. From El Amki and Wegener, 2017.